One crucial component of the aquarium hobby that helps the hobbyist in his/her venture is knowledge. Aquarium keeping is an interesting hobby in that it combines principles from physics, chemistry, and biology. The more we understand each of the principles within these disciplines, the easier our hobby becomes. Of course, biology is the most understood of the three fields, because lets face it our final goal is to maintain living creatures. However, much like junior year in high school, we need to understand a basic level of chemistry to survive (or for our fish to survive as the case may be).
In this series of articles, I hope to put many of the chemical principles behind aquarium keeping into laymen’s terms. In future articles I will discuss subjects such as the nitrogen cycle, dissolved oxygen, and pH. The more we understand each of these factors in water chemistry and why they are good or bad, the more we understand our hobby as a whole. This issue’s installment is going to start at the beginning: water. Water is such a basic element of the aquarium hobby that it is often overlooked. However, it is no coincidence that water is the molecule which makes up the oceans, lakes, and rivers from which the fish we keep originate. It is because of the way that water interacts with other chemicals, itself, and our fish that the majority of life on this planet is sustained within this amazing matrix.
Water: The Molecule
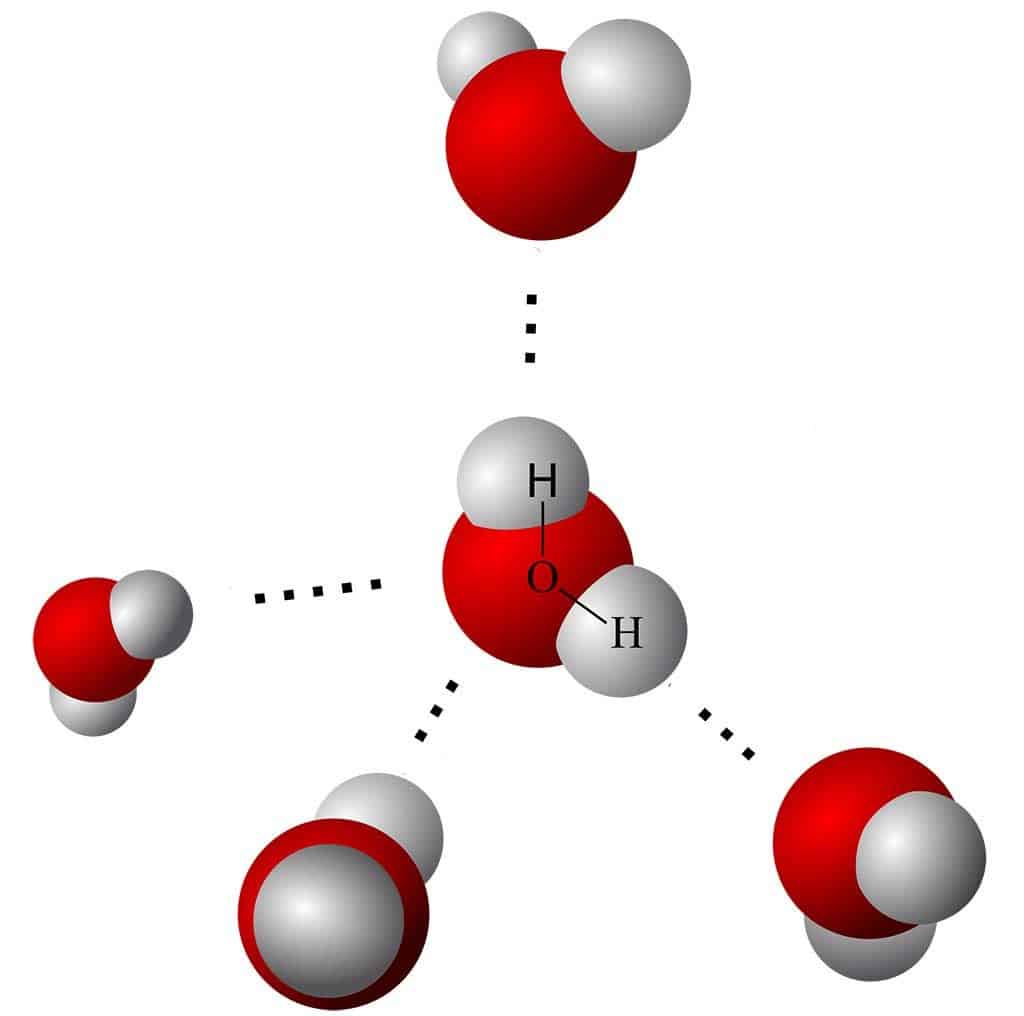
Water is a molecule unlike any other. It is made up of one oxygen molecule and two hydrogen molecules. Each hydrogen forms a bond connecting it to the oxygen molecule, forming an “upside-down V” shape. If you look at the upside-down V shape of water, you will notice that the two hydrogen atoms (which have an overall positive charge) are positioned across from the oxygen atom (which has an overall negative charge). This alignment means that one side of the water molecule (the oxygen side) has a slight negative charge and vice versa. Conversely, each side will then attract molecules or ions of opposite charge. This is known as the dipolar nature of water.
At this point you’re probably having high school flashbacks, because: “Why in the world did I need to know all that, and what will I ever use it for!?!?!?!”
Water’s dipolarity is exactly what makes it such a great solvent for the laundry list of chemicals with which we aquarists worry ourselves: nitrate, ammonia, oxygen, iron, calcium, etc. All of these molecules and ions have one thing in common: they have a charge (the charge can even be a partial charge like water’s). Imagine, in your mind’s eye, a lone Calcium ion that was just dissolved into your reef aquarium by a calcium reactor. The Ca2++ has a positive charge, so when a group of water molecules encircle the calcium ion with their negative ends pointing in…Voila! The calcium ion has been dissolved.
This brings up a vital factor to the well being of your fish. Due to the way that it must organize itself in order to dissolve a chemical, water has a finite amount of space available for dissolving. Often times nitrates are brushed aside as being not dangerous to freshwater fish. However, a tank which has been ignored and allowed to build up nitrates will have significantly “less space” to dissolve critical molecules like oxygen or carbon dioxide.
Temperature
Water’s ability to dissolve is also determined by its temperature. Aquarists are interested in dissolving chemicals in every state of matter, whether it is a solid, liquid, or gas. As water temperature increases, solids and liquids tend to dissolve more readily. However, as temperature decreases, gases tend to dissolve more readily, which makes a real balancing act out of maintaining water temperature. In every aquatic environment gases, liquids and solids are essential to fish health. If you think about it, there are really very few fish that require temperatures above eighty degrees. Now you understand why keeping dissolved wastes down is so important when keeping discus at the warm temperatures they enjoy (well, actually that’s only one of many reasons). When phosphates build up due to a poor water change regimen and overfeeding, the amount of phosphate dissolved in the aquarium directly decreases the amount of “free room” water has to dissolve oxygen for the discus to breathe. Oxygen depletion has many deleterious effects on all fish, but the discus, which has evolved in oxygen-rich streams, will be doomed in water unable to dissolve much oxygen.
Saltwater
Those of us who keep marine aquariums are especially affected by the dipolar nature of water. One of the questions I was fielded the most as a pet store, fish clerk was: “Is saltwater really as hard as they say it is?” Now I could have delivered this article in a brilliant oratory transcription, but a) the customer was probably just curious b) “yes” is a whole lot quicker. In fact, the reason that “saltwater is as hard as they say it is”, is due to water’s dipolarity. Saltwater, of course, must first dissolve a series of salts to fulfill the proper salinity before it can begin to dissolve other essential molecules or ions. While most of the people who approached me about starting a saltwater would consider the proper maintenance behind a marine aquarium overbearing, the knowledgeable hobbyist has a number simple of methods at his/her disposal to keep the tank free of excess waste molecules. Protein skimmers, macro-algae cultivation, and deep sandbeds are just three methods aquarists use to remove excess, waste byproducts.
I personally have a 29-gallon mini-reef aquarium, which has been a constant bear; because of saltwater’s reduced ability to dissolve essential molecules. A 65W power compact light fixture and a 20W normal output light fixture light the tank. This amount of light raises the temperature into the mid-eighties, at which point all the corals begin to bleach from insufficient dissolved oxygen. I use a fan to keep the temperature down, but this causes a great deal of evaporation. The confines of my small, one-room apartment prohibit an automatic top-off system, thus requiring me to manually top-off the aquarium with kalkwasser throughout the day. This is merely one obstacle in the three-ring circus balancing act that is my mini-reef aquarium.
Osmosis
All of these ions and molecules that we are dissolving in water don’t just “sit there”. They are constantly moving so that they are equally dispersed throughout a body of water. Imagine a ten-gallon aquarium, filled with distilled water, divided into two compartments with a piece of steel. If only one side was adjusted to a salinity of 33, as soon as the piece of steel is removed, the salt ions would slowly distribute themselves so that the entire aquarium registered a salinity of 16.5 (exactly half of 33). This phenomenon is called diffusion.
All of the salt ions, bouncing off of the steel divider, were exerting a certain amount of pressure on the piece of steel, specifically called: osmotic pressure. The higher the salt concentration, the greater the osmotic pressure. Steel is an incredibly impermeable material, but our fish’s cellular membranes are not. In order to prevent a virtual implosion, our fish’s cellular membranes are actually permeable to small ions and water. This means that if the water outside of a fish’s cells has more dissolved ions than the water inside of its cells (i.e. osmotic pressure is greater outside of the fish’s cell); the fish’s cell membrane allows small ions to diffuse into the cell and water to exit the cell until the osmotic pressure is equalized. Every living creature that makes water its home has to be able to deal with osmotic pressure in this manner. Although we take it for granted, this is probably the cell’s membrane most important function.
After thousands, if not hundreds of thousands, if not a million years, of adaptation, all aquatic creatures are used to a certain amount of natural fluctuation in osmotic pressure. This is another illustration of why saltwater is deemed: “harder than freshwater”. Since the vast volume of the ocean provides for pretty consistent water parameters, corals, sea stars, butterfly fish, etc. are very intolerant of changes in salinity, pH, etc. However, some freshwater fish actually require relatively drastic changes in water parameters to breed (some goes as far a requiring no water for a short period of time, ala many killifishes). Nonetheless these changes happen within a certain range, and due to years of evolution, anything outside of this range is often lethal.
Hopefully all of this painful chemistry sheds a little more light on the “fish-keeping experience”. We now know that water’s dipolarity makes water one of the best solvents on earth, however, with a limited capacity. We’ve also learned that the more molecules that water has to dissolve, the less space water has to dissolve essential ions and molecules for our fish. The higher the water temperature, the more readily solids and liquids will dissolve, while conversely limiting how much gas can be dissolved. Finally, as the molecules and ions that water dissolves move about, they exert a certain amount of pressure on our fish called osmotic pressure. Stay tuned for the next episode, when I will cover everything you ever waned to know about conductivity and water hardness. These two water parameters measure how many molecules and ions are dissolved in water, and directly reflect the amount of osmotic pressure exerted upon our fish. In the meantime let’s keep learning about and caring for our fish.
First publication: Fincinnati, Greater Cincinnati Aquarium Society
Source: Aquarticles.com (No longer available)
